A cation is a molecule with a positive charge.
One of the most important properties of colloids is their ability to adsorb, hold, and release the mineral nutrients and water molecules which are so important to the plant.
In horticulture we frequently talk about Cation Exchange Capacity (CEC). This is a useful indicator of soil fertility because it shows the soil’s ability to supply three important plant nutrients: calcium, magnesium and potassium.
What CEC actually measures is the soil’s ability to hold cations by electrical attraction. As noted, cations are positively charged elements, the positive charge indicated by a + sign after the element symbol.
The number of + signs indicates the amount of charge the element possesses.
The five most abundant exchangeable cations in the soil are calcium (Ca++ ), magnesium (Mg++), potassium (K+), sodium (Na+) and aluminium (Al+++).
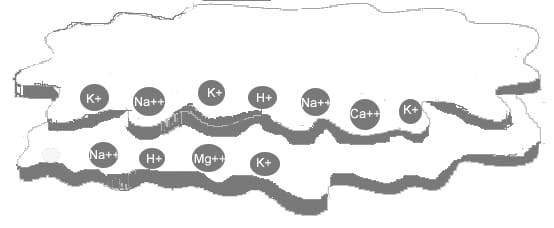
Cations are held by negatively charged particles of clay and humus called colloids. Colloids consist of thin, flat plates, and for their size have a comparatively large surface area. For this reason they are capable of holding large quantities of cations and in effect act as a storehouse of nutrients for plant roots. As plant roots take up cations, other cations in the soil water replace them on the colloid.
If there is a concentration of one particular cation in the soil water, those cations will force other cations off the colloid and take their place. The stronger the colloid’s negative charge, the greater its capacity to hold and exchange cations, hence the term cation exchange capacity (CEC).
Below is a diagrammatic representation of the flat plate-like structure of a colloid (either humus or clay). Its negative charges are along the edges of the plates. The cations are attached to the colloid by electrical attraction between the positive and negative charges.
Note: Here we have written out the cations to show the number of positive charges in full, e.g. Al+++, but often a shorthand is used, e.g. Al+3.
CEC measurement
Concentrations of cations are expressed in centimoles of positive charge per kilogram of soil (cmol(+)/kg). Adding the concentrations of each cation gives you an estimate of the CEC figure. A figure above 10 cmol(+)/kg is preferred for plant production. Soils with high levels of swelling clay and organic matter can have a CEC of 30 cmol(+)/kg or more.
There are five exchangeable cations, and the percentage of each one is shown in soil test results as a percentage of CEC. The desirable ranges for them are: calcium 65 – 80% of CEC, magnesium 10 – 15%, potassium 1 – 5%, sodium 0 – 1%, and aluminium 0%.
pH and CEC
Soil pH is important for CEC because as pH increases (becomes less acid), the number of negative charges on the colloids increase, thereby increasing CEC.
CEC levels
Humus
CEC varies according to the type of soil. Humus, the end product of decomposed organic matter, has the highest CEC value because organic matter colloids have large quantities of negative charges. Humus has a CEC two to five times greater than some types of clay, and up to 30 times greater than others, so is very important in improving soil fertility.
Clay
Clay has a great capacity to attract and hold cations because of its chemical structure. However, CEC varies according to the type of clay. Some types of clay can hold on to many nutrients. These clays can also absorb a lot of water. They may take on so much water that each clay particle almost doubles in size. In contrast, heavily weathered clays, known as kaolinite clays, have a relatively low CEC. They do not have the ability to hold on to many nutrients or absorb a lot of water.
Sand
Sand has no capacity to exchange cations because it has no electrical charge. This means sandy soils have very low CEC, but this can be improved by adding organic matter.
Aluminium and Sodium
Aluminium (Al+3) and sodium (Na+2) cations are not plant nutrients, so are not wanted by the plant. Aluminium is not present as a cation when soil pH is over 5 because it is precipitated out of the soil solution. It is only at pH levels below 5 that it may become available as a cation, and under 4.5 may become available in toxic levels, displacing other cations from the clay or humus colloids. This is one reason why it is important to maintain pH levels at 5.0 or more.
When exchangeable sodium is present in quantities greater than about 5% of the CEC, it makes the clay particles unstable in rainwater. This shows up as dispersion or cloudiness in water. Dispersive soils have poor drainage and set hard on drying.
Leaching
If a soil has a low CEC and high sodium levels, up to half of the important plant nutrient cations may be in the water around the soil particles, and not actually held by colloids (because the colloids are taken up with sodium cations). The plant nutrient cations are then very susceptible to being leached or drained away in the soil water. Soils with a high CEC have a much lower percentage of cations in the soil water, so are far less susceptible to nutrient loss by leaching.
Improving CEC
You can improve CEC in weathered soils by adding lime and raising the pH. Otherwise, adding organic matter is the most effective way of improving the CEC of your soil. This can be done with permanent pasture, green manure crops, leaving crop stubbles to rot, rotating crops or pasture, and the addition of mulch and manure.
Cation exchange is constantly going on in soils and is of great importance for the grower. Without some mechanism to temporarily hold cations in the soil, plants would be unable to obtain sufficient quantities of the essential nutrients to grow. Without cation exchange, the nutrients would simply be leached downward in the soil and lost.
Cation exchange plays a role in other soil processes as well. Acidification is the process of exchanging basic cations, such as Ca+2, Mg+2, K+, and Na+, for acidic cations, such as H+ and Al+3. Liming acid soils results in a reversal of this process, H+ ions are exchanged for Ca+2 ions. If cationic fertiliser nutrients are not held by the soil colloids, they will be lost by leaching as water percolates through the soil.
The CEC and the balance of ions are important to horticulturists because they determine such important features as the ability of a soil to replenish some nutrients to the soil solution, from where they are taken up by the plants, and the pH (acidity or alkalinity) of the soil.
Science and the Garden, Ingram et al.


Inorganic colloids, how do they differ from organic colloids and what effect do they have on soil reactions and on plant growth ?